
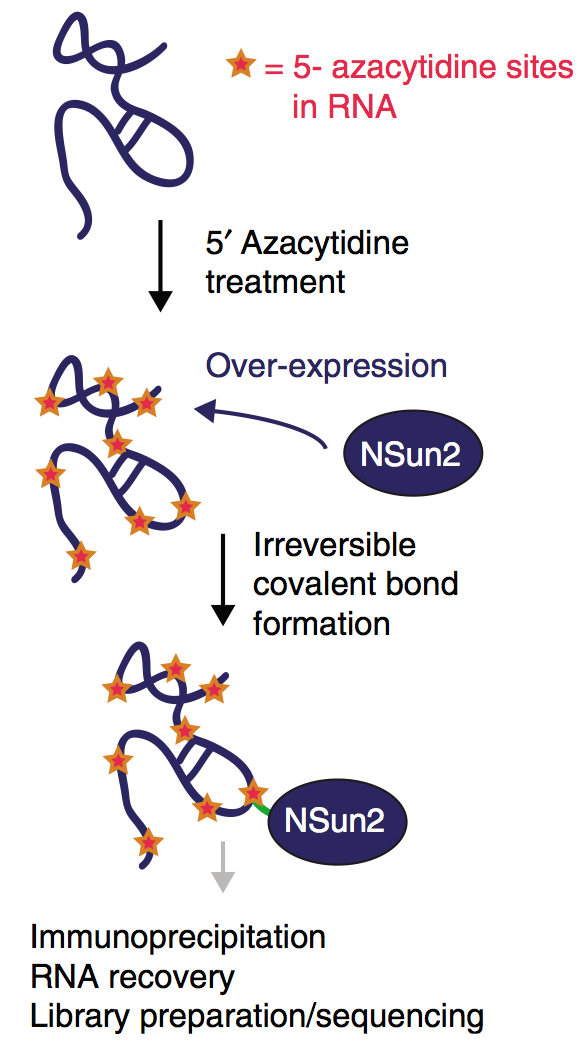
The beads were washed 3x with 1 ml ‘PNK+EDTA’ buffer. Next, 1 µl of 10 mM ATP was added to each sample and the reaction mix was 80 µl of the mix was added to each sample and incubate in Thermomixer at 37C for 10 The beads were washed 3 times with 1ml of 1x PNK buffer. atīeads were incubated in the Thermomixer at 16C over-night (1000 rpm every 5 min Followed by 40 µl of ligase mix (with final 元 conc. The 1x PNK buffer was carefully discarded from the beads and 40 µl of the linker mix Next, the beads were washed twice with 1 ml of 1x ‘PNK+EDTA’ buffer and twice Beads were incubated at 37C for rpm every 3 min for 15 s. The last 1x PNK wash was removed from the beads and CIP mix was applied. The over-digested sample is left at 4 C in PNK buffer until PNK treatment step.Ĩ µl 10x dephosphorylation buffer (Roche)ģ µl alkaline phosphatase (Roche, 10713023), kept at 4C Next two steps: CIP treatment and 3’ RNA linker ligation are performed only with sample treated with low RNase concentration (1:5000). Next, the supernatants were removed and beads washed twice with 1 ml of lysis bufferĪnd twice with PNK buffer, the over-digested sample was resuspended in PNK buffer. Beads/lysate mix was rotated for 1 hr at 4C. Supernatants were transferred onto Invitrogen magnetic Dynabeads protein G coated Lysates were centrifuged at 20800 g in Eppendorf centrifuge 5414R for 20 min at 4C. Next, 3 µl of SUPERase In (Ambion, 2U/µl) was added to each duplicate, in order to 20 µl of each RNase T1 dilution was added to one of the duplicate eppendorfs, and Dilutions of RNase T1 (Ambion, 1000U/µl) at 1/100 (overdigested sample) and atġ/5000 (underdigested sample) were prepared in the lysis buffer. 50 µl of DNAse I (RNase free, 1U/µl, Epicentre Biotechnologies) was added to eachĮppendorf, followed by incubation at 37C for 5 min, 1000 rpm in Thermomixer. Cells were lysed for 30 min on the rotating wheel at 4C. Each of cell pellets was resuspended in 1ml of lysis buffer (FLAG IP kit, Sigma),Ĭontaining proteinase inhibitor cocktail, EDTA-free (Roche), 0.1% SDS and This was rotated at room temperature for 30-45 min. These were re-suspended in 60 l 0.1 M Na-phosphate pH 8.1 and 20 l of antibody. Beads were washed 3x with 0.1 M Na-phosphate, pH 8.1. 20 l of Dynabeads protein G (Invitrogen) was used for each eppendorf of cross-linked Snap -frozen at -80˚C until use (each eppendorf contained ~200 µl of cells). The cells were harvested and pelleted at 4C, the pellet was washed with ice-cold PBS, Tray with ice on the bottom of the Stratalinker 2400 (STRATAGENE) and cells were Was added to keep the cells moist (~10ml/ plate area). The cells were rinsed once with Dulbecco’s PBS (Ca2+ The cells were fed the day before the experiment or split if needed. An adequate number of cells were seeded into 150 mm dishes to achieve a culture ofĪbout 70-80% confluence on the day of UV treatment. Protease Inhibitor Cocktail, EDTA-free (Roche) Schematic representation of main steps in CLIP method.ĭetailed description is given in the text.įrom FLAG Immunoprecipitation Kit cat no. CLIP tags were then amplified by RT-PCR, cloned, sequenced and analysed.įigure 2.2.
#ICLIP TECHNIQUE FREE#
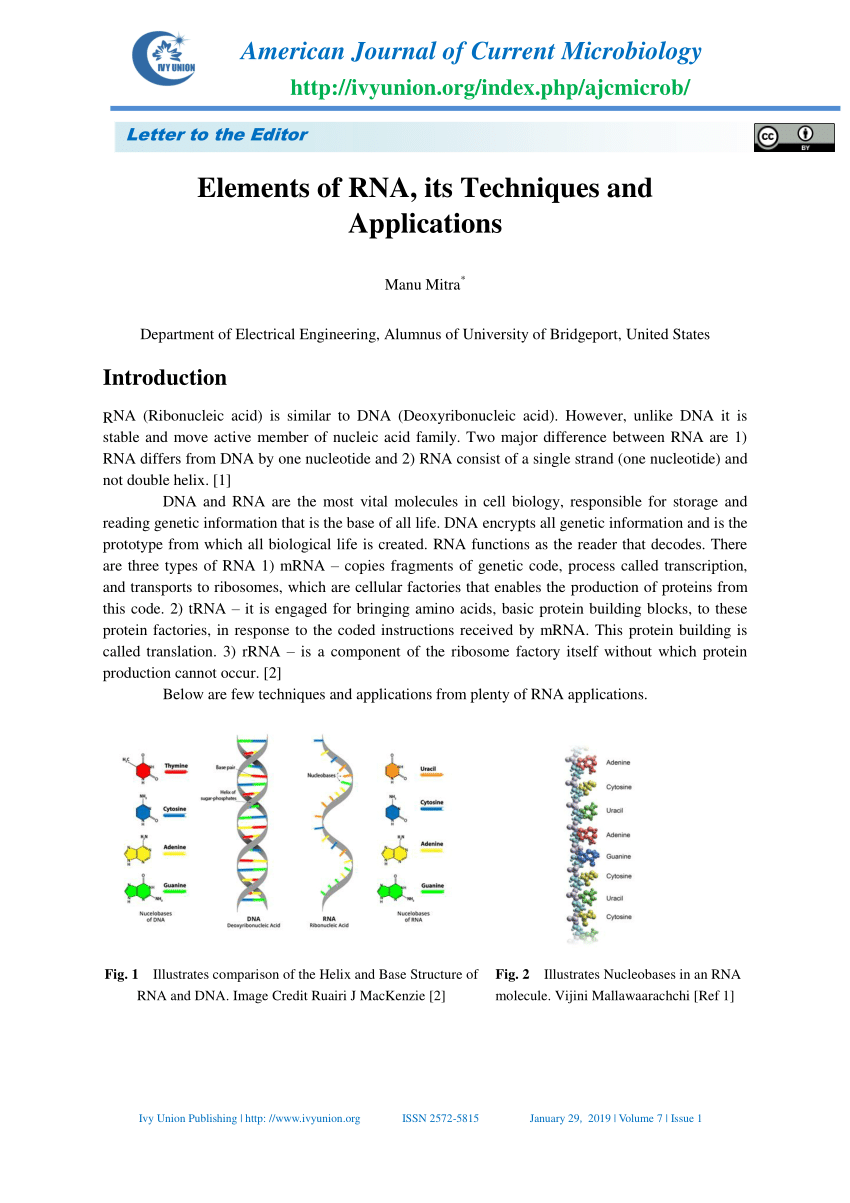
Protein was digested by proteinase K and 5’ RNA linker was ligated to free RNA. Thin region of the membrane corresponding to protein–RNA complexes ofĪppropriate size was localized by expose to X-ray film and cut out. SDS-PAGE electrophoresis of protein-RNA complexes and transfer to a) Dephosphorylation of RNA.Ĭ) Radioactive labelling of RNA by γ 32P on the 5’ terminus. Immunoprecipitation of protein-RNA complexes. Partial digestion of RNA after cell lysis. Cells grown in 150 mm plates were UV-irradiated on ice leading to formation of a Presented only main steps of the method, followed by a detailed protocol used to
The protocol for CLIP assay was followed as described in Ule et al., 2005.


 0 kommentar(er)
0 kommentar(er)
